Abstract
Adverse events (AEs) associated with blood component transfusion have been widely surveyed. In contrast, surveillance of AEs associated with hematopoietic stem cell (HSC) infusion in HSC transplant (HSCT),including bone marrow transplant (BMT), peripheral blood stem cell transplant (PBSCT), and cord blood transplant (CBT),has been less rigorous, even though HSC products contain cells of diverse maturity and viability,plasma with various antigens, cytokines and antibodies, and dimethyl sulfoxide (DMSO) in the case of cryopreserved products. In fact, HSC infusion is associated with several AEs, e.g., allergic reactions, flushing, hypo- or hypertension, and respiratory distress, which have been attributed to toxicity of dead cells and DMSO (Otrock et al, Transfusion, 2017). However, our recent prospective surveillance revealed that HSC infusion-related AEs often occurred in each HSC type and the overall rates of AEs were greater in allo-BMT with no DMSO, compared with auto-PBSCT, allo-PBSCT, and allo-CBT typically cryopreserved with DMSO. Hypertension was the most common AE in each HSC source, with the highest rate in BMT, while allergic reactions were the most frequent in allo-PBSCT. A multivariate analysis identified a history of transfusion reactions as a risk factor of HSC infusion-related AEs (Ikeda et al, Transfus Med Rev, 2018). These findings suggest that some DMSO-independent factor(s), such as plasma components, may contribute to HSC infusion-related AEs.
Thus, we asked if HSC volume and component effects were more substantial in small recipients, and if age-related factors alter susceptibility to HSC infusion-related AEs in pediatric patients. So far, data on HSC infusion-related AEs in pediatric and low body-weight recipients are lacking. Here, to address these issues, we investigated AEs due to HSC infusions in 219 recipients of <45 kg body weight, including 90 recipients 15 years old or younger, versus data from 1,125 recipients in general (general recipients)in the prospective study described above.
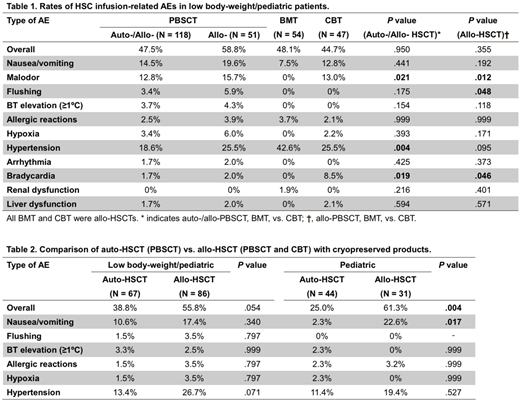
The rates of overall HSC infusion-related AEs were quite similar among HSC sources among low body-weight/pediatric recipients (Table 1) andexclusivepediatric recipients (P= .158 in auto-/allo-HSCT and .867 in allo-HSCT), in contrast to general recipientswhose rate of AEs was highest in BMT. In addition, bradycardia was more often reported in CBT compared with PBSCT and BMT (Table 1), especially in pediatric recipients (30.8% in CBT and 0% in others, P< .001). On the other hand, there were some similar trends between low body-weight/pediatric recipients and general recipients: PBSCT and CBT recipients, but not BMT recipients, complained of malodor, whereas the rate of hypertension was highest in BMT (Table 1).
Next, we compared HSC infusion-related AEs between auto- and allo-HSCT using cryopreserved products (Table 2). This comparison showed higher overall rates of AEs in allo-HSCT compared with auto-HSCT, especially in pediatric recipients, suggesting that plasma componentsrather than DMSO contribute to HSC infusion-related AEs in low body-weight/pediatric recipients as well as in general recipients. Of note, pediatric recipients showed a 10-foldhigher incidence of nausea/vomiting in allo-HSCT versus auto-HSCT, instead of allergic reactions, for which the incidence was significantly higher in allo-HSCT than auto-HSCT in general recipients.
Subsequently, we sought factors that correlate with HSC infusion-related AEs in allo-HSCT using multivariate analysis with logistic regression, which identified lymphoid neoplasms over myeloid neoplasms as a factor for overall AEs (OR 3.013, P= .026), while history of transfusion reactions did not reach statistical significance (OR 2.368, P= .066). Notably, in a multivariate analysis for any grade ≥2 AEs, there were some factors that did not correlate with general recipients but did with low body-weight/pediatric recipients, including complications before HSCT (OR 5.764, P= .019), without plasma or red blood cell removal for ABO mismatch (OR 3.815, P= .032), and >10 ml/kg infusion volume (OR 5.306, P= .027).
In conclusion, our data quantifysome specific symptoms associated with HSC infusionin low body-weight and pediatric recipients. We should be mindful ofinfusion volume and preexisting complications when small recipients receive HSC infusion.
Fujiwara:Astellas: Consultancy; Kyowa-Hakko: Consultancy; Kirin: Consultancy; Chugai: Consultancy; Pfizer: Consultancy; Shire: Consultancy. Muroi:JCR: Speakers Bureau; Becton: Speakers Bureau; Dickinson and Company: Speakers Bureau; Japanese Red Cross Society: Speakers Bureau. Mori:Astella Pharma: Honoraria; Kyowa Hakko Kirin: Honoraria; Japan Blood Products Organization: Honoraria; MSD: Research Funding; Ono: Honoraria; Novartis Pharma: Honoraria; Eisai: Honoraria; Janssen: Honoraria; Asahi Kasei: Research Funding; Novartis Pharma: Research Funding; Celgene: Honoraria; Taisho Toyama Pharmaceutical Co: Honoraria; Shire Japan: Honoraria; Pfizer: Honoraria; CHUGAI: Honoraria; MSD: Honoraria; SHIONOGI: Honoraria. Nagai:Ono Pharmaceutical Co.Ltd.: Consultancy; Kaneka Corporation: Research Funding; Kawasumi Laboratories Inc.: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal